Communication
Our offer
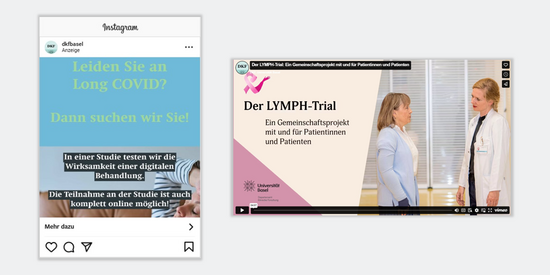
Recruitment of study participants
Recruiting study participants is one of the biggest challenges in clinical studies. To ensure that your project does not fail at this point, we offer tailored communication strategies to overcome this hurdle.
We identify the most effective print and online channels, manage the creation and distribution of informational materials, coordinate all parties involved and handle communication with external partners.
Whether it is a video, social media post or Google ad, we help you in reaching your target audience directly. This ensures that potential participants become aware of your study and that you meet your recruitment goals.
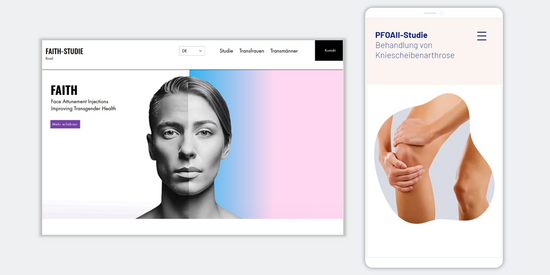
Websites for clinical studies
With a website specifically created for your study, you will not only reach potential participants but also their relatives and healthcare professionals. Our team develops websites tailored to your study and communication goals. We focus on:
- User-friendly and visually appealing design
- Multilingual content
- Scientifically grounded content tailored to your target audience (texts in lay language, videos, photos, graphics)
- Search engine optimisation
- Compliance with data protection regulations
- Evaluation of website usage
Newsletter for study sites
In multicenter studies, effective communication with the participating study sites is an important success factor. We design and send professional newsletters on your behalf, allowing you to distribute information and documents to the study sites involved.
Communication of study results
Your research findings deserve proper visibility. To maximise the impact of your study both within the scientific community and among the general public, we offer support with:
- Preparing your study results for specific target audiences (social media posts, videos, articles)
- Disseminating them through our own channels (social media, newsletter, website)
- Communicating your results to relevant communication offices
Regulatory requirements
Our team ensures that all communication measures comply with the applicable regulatory and legal requirements. We pay particular attention to data protection compliance, consideration of ethical standards and compliance with regulatory requirements when advertising clinical studies.
Lay summaries
Lay summaries are comprehensible summaries of study results intended for the general public. They are written in laymen's language and must be published in the respective national language. Lay summaries are legally required for all clinical studies (Art. 64, Art. 65a, Annex 5 ClinO; Art. 41 and 42 ClinO-Mep). Our team creates easy-to-understand lay summaries in compliance with the guidelines of Good Lay Summary Practice (GLSP).

Do you need to know which regulatory requirements apply to recruitment materials?
Recruitment materials generally have to be approved by the ethics committees. Swissethics offers helpful checklists and templates for this purpose:
Would you like to optimise participant recruitment?
We have compiled a catalog of measures and would be happy to advise you.
- Preparation of study results tailored to the target group and dissemination via the DKF's communication channels (social media, newsletter, website)
- Communication of study results to relevant institutions
