Latest publications
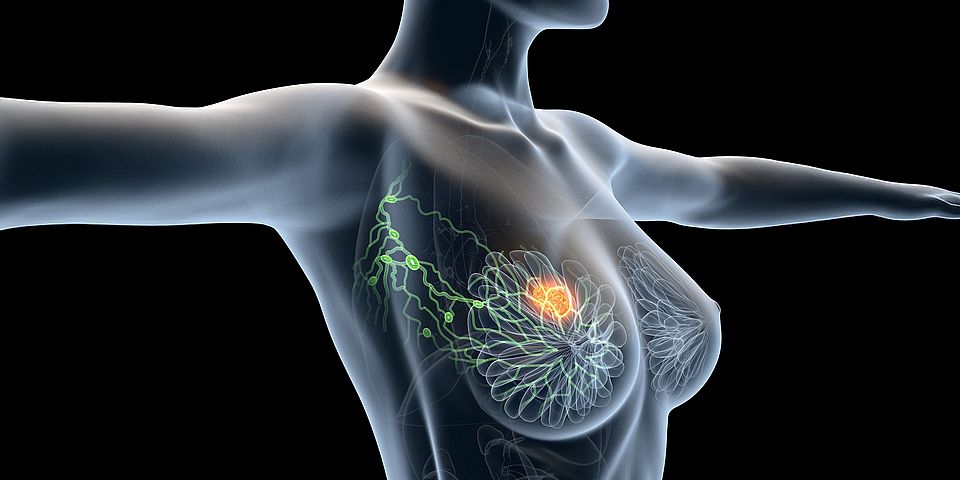
FG Walter P. Weber: Montagna G, et al. Oncological outcomes with and without axillary lymph node dissection in patients with residual micrometastases after neoadjuvant chemotherapy (OPBC-07/microNAC): an international, retrospective cohort study
FG Urs Frey: Da Silva Sena CR, et al. Markers in Infants of Mothers With Asthma and Associations With Respiratory Outcomes
FG Jasper Boeddinghaus: Boeddinghaus J, et al. Cardiovascular Risk Across Myocardial Injury and Infarction Categories Using the Universal Definition: An Individual Patient-Level Data Meta-Analysis
FG Nicole Probst-Hensch: Brugger C, et al. Changes in psychological distress during conflict escalation in an adult population-based cohort in the Gaza Strip (2020-2025): a longitudinal analysis
FG Bernice Elger: Milford SR, et al. Promoting xenomorphic patient-facing AIs: The case against anthropomorphism in medical AIs
Events
11 February 2026, 16.00-18.00, Centro
PhD Satellite Event
More information
12 February 2026, 11.15-17.15, ZLF
Clinical Research Day 2026
More information
5 March 2026, 14.00-17.00, University of Basel
REDCap® Workshop: How to develop your own database
More information
12 March 2026, 6 days, 13.00-17.00, DKF
Reading and Writing Statistics in Clinical Publications
More information
starting 21 April 2026, 2 days, 09.00-17.00, University Library Basel
Practical Strategies for Effective Research Data Management
More information
starting 22 April 2026, 3 days, University of Basel
Applied Statistics using R: Analysing Medical Data
More information
28 April 2026, 09.00-13.00, DKF
Gemeinsam für Forschung, die uns weiterbringt
More information
Looking for more news?
SUBSCRIBE TO NEWSLETTER