Patient and Public Involvement - why and how?
What is Patient and Public Involvement (PPI)?
This term covers all activities that enable patients to be actively involved in the planning, execution and evaluation of new research projects. The aim is to shape research with or by instead of at , about or for those affected (1) and to theerfore to let them participate as equal partners. PPI contributors can also be representatives from patient organisations, relatives and caregivers or other interested members of the public.
Mere participation in a clinical trial or human research project is not considered PPI because individuals in this role participate in research but do not actively shape it. Synonymous terms for PPI are "patient-focused drug development" or "patient engagement."
Why PPI?
The importance of involving and giving a voice to those affected is obvious from an ethical perspective. At the same time, PPI activities have the potential to make clinical research processes more efficient and research results more useful. PPI representatives have unique knowledge, perspectives, and experiences that can influence the relevance, quality, appropriateness, and credibility of clinical research (2). For example, evidence suggests that recruitment improves when individuals who themselves have experience with the disease under investigation have been involved (3).
In addition, researchers seeking fundingareincreasingly encouraged to incorporate PPI activities in the planning, execution, translation and evaluation of their research projects. For example, the Investigator Initiated Clinical Trials (IICT) funding program of the Swiss National Science Foundation (SNSF).
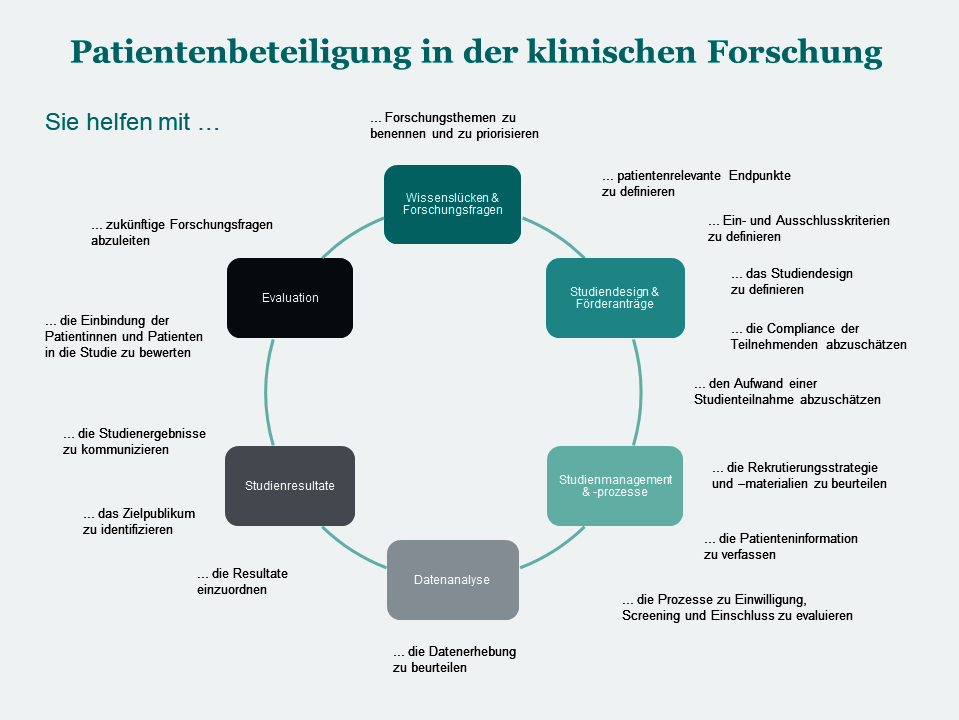
Which PPI activities are suitable for my project?
The choice of appropriate PPI activities, which type of PPI contributors to be involved and timing of PPI activities may vary depending on the objectives in each trial. In any case, thought should also be given to the possibilities for involving stakeholders early in the planning phase of a research project. The following graph shows various possibilities:
You can also find concrete opportunities for participation, as well as decision-making aids for choosing PPI representatives and types of activities, in our PPI Guideline and PPI SOP.
As a general rule, mutual trust and a transparent, partnership-based approach are a prerequisite for a fruitful dialog between researchers and stakeholders.

Documents & Instructions
- Guide for researchers to address patient and public involvement (PPI) in clinical trials, SCTO (PDF, 346.82 KB)
- PPI Fact Sheet, SCTO (PDF, 131.20 KB)
- Guideline on Patient Involvement in Clinical Research at the Department of Clinical Research, University of Basel (PDF, 313.26 KB)
- Remuneration Policy for Patient and Public Involvement (PPI) Activities, SCTO (PDF, 313.97 KB)
- Standard Operating Procedure (SOP) Patient Involvement, DKF