6 May 2024
Flexible expert staff: the DKF helps out with qualified study nurses
Conducting a clinical trial is labor-intensive. Well-trained and experienced staff is a prerequisite for smooth and efficient processes during this phase. On request, the DKF supports study teams with qualified study nurses.
Intensive project phase
Once the study protocol has been finalised and a positive ethics vote is on the table, things have to move quickly. Suitable patients have to be found and convinced to participate, the study medication has to be organised, study visits have to be arranged and Case Report Forms filled out.
This phase of a clinical trial is particularly labor-intensive and the availability of sufficient expertise, experience and time in the study team can be crucial. Not all study teams have the opportunity to hire additional staff. Especially, if it is not certain whether further projects will follow that can cover the personnel costs.
Flexible offer
In such cases, the DKF can help out. The team «On Site Management» is mandated with sufficient personnel resources to support research groups. The assignments of trained study nurses can be offered very flexibly: Short-term vacation and sick leave replacements or long-term commitments with commencement of overall responsibility for setting up, conducting and completing a study. The DKF study nurses are assigned to the departments or clinics of the study teams or carry out their tasks at the DKF Outpatient Study Centre (ASZ), depending on what best suits the processes and spatial conditions.
Qualified study nurses make a significant contribution to the successful conduct of clinical studies. Requests for support can be made at any time using the contact form.
For further information:
Silke Scarascia
Managing Study Nurse
E-Mail
Phone +41 61 328 77 09
Request for study nurse support:
Contact form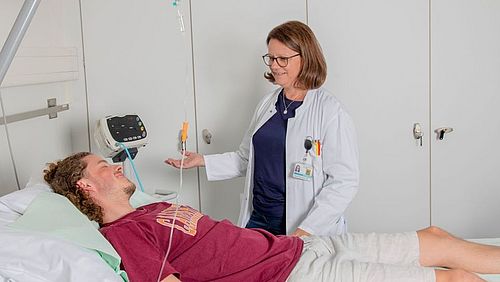
Service
«On Site Management»
Competencies of study nurses at the DKF
- Management of participants
- Screening and inclusion
- Planning and management of study visists
- Conducting surveys (paper, online, telephone)
- Study medication
- Ordering, preparation, administration, disposal
- Sample management
- Collection, preparation, dispatch, storage
- Documentation
- Completion of Case Report Forms (CRFs)
- Safety management
- Drug Accountability
- Maintaining Invesitgator Site File (ISF) and Trial Master File (TMF)
- Preparing and accompanying monitoring visits
- All workflows in accordance with Good Clinical Practice (GCP) and the applicable Quality Management System (QMS)
